Tuesday, September 17, 2013
Sunday, September 15, 2013
But when will Mumbai Eye hosps and large clinics will have neuro-ophthalmologists ....Alok
worst is in Mumbai most do not know if neuro ophthalmologists super super specialty exists. In USA they are everywhere and in India Sankar Netralaya has 5 of them ......Alok
North Shore Eye Care Adds Ophthalmologists Lawrence Buono, MD Christine Speer-Buono, MD To Medical Staff
In their continuing effort to expand their comprehensive eye care services, the doctors and staff of North Shore Eye Care proudly welcome Lawrence M. Buono, M.D., a Fellowship-Trained Neuro Ophthalmologist who trained at the world-renowned Wills Eye Institute in Philadelphia, PA and his wife, Christine Speer-Buono, MD a general ophthalmologist.
Southold, Long Island (PRWEB) September 12, 2013
In their continuing effort to expand their comprehensive eye care services, the doctors and staff of North Shore Eye Care proudly welcome Lawrence M. Buono, M.D., a Fellowship-Trained Neuro Ophthalmologist who trained at the world-renowned Wills Eye Institute in Philadelphia, PA and his wife, Christine Speer-Buono, MD a general ophthalmologist.
Dr. Buono is a New York native who grew up in the area and enjoys the opportunity to continue practicing on the east end of Long Island. “I am honored by the opportunity to join the medical staff of such a progressive and established eye center as North Shore Eye Care,” said Dr. Buono, who along with his wife have been practicing on the east end of Long Island for nearly nine years. “North Shore Eye Care has a rich tradition of excellence on Long Island and our philosophies on patient care are a perfect fit.”
In addition to Dr. Buono’s extensive experience in treating complex ocular and neuro-ophthalmic conditions, he also has considerable experience with implanting presbyopia-correcting intraocular lenses and treating complicated cataract cases. He currently serves on staff at Southampton Hospital, Eastern Long Island Hospital and Peconic Bay Medical Center.
“I love the challenge of treating complex cataract and neuro-ophthalmic conditions,” said Dr. Buono, “but also thoroughly enjoy utilizing all of the breakthroughs in lens implant technology, diagnostics and surgical techniques to help improve our results in standard cataract surgery.”
Dr. Buono completed his medical degree at Thomas Jefferson Medical College. After completion of a medical/surgical internship at the Presbyterian Hospital/ University of Pennsylvania, he returned to New York for his ophthalmology residency at New York Medical College in Valhalla. Upon completion of his residency, he completed a neuro-ophthalmology fellowship at the Wills Eye Hospital in Philadelphia. Dr. Buono then served as an Assistant Professor of Ophthalmology at Duke University in the neuro-ophthalmology and comprehensive ophthalmology divisions and has authored numerous papers in the ophthalmic literature, and has educated medical students, residents, and fellows.
“It is rare to have a physician of Dr. Buono’s training and expertise join our medical staff,” said Jeffrey Martin, MD. “With Dr. Buono’s training and expertise, we feel confident that our level of comprehensive eye care that we now offer out of our six Long Island locations meets or surpasses the services found in the leading eye centers in the world.” Dr. Buono and Dr. Speer-Buono will be practicing in the Southold, Riverhead and Southampton offices of North Shore Eye Care.
Also joining North Shore Eye Care’s medical staff is Dr. Buono’s wife, Christine Speer Buono, MD, FACS. Dr. Speer Buono graduated from Vanderbilt University with a degree in Chemistry and Spanish, then returned to her home state of Arkansas to receive her medical degree and graduate first in her class from the University of Arkansas for Medical Sciences. She completed an internship at Georgetown Hospital/ INOVA Fairfax Hospital and then residency at the Wills Eye Hospital in Philadelphia, PA. After her residency completion, Dr. Buono became an Assistant Clinical Professor of Ophthalmology at the Duke Eye Center where she practiced comprehensive ophthalmology including cataract surgery.
She also educated medical students and residents through lectures, clinics, and surgical staffing. Since that time, Dr. Buono has enjoyed being a part of this outstanding practice on the East End. Her interests include cataract surgery, laser surgery for glaucoma and after cataract surgery, dry eye, glaucoma, herpetic eye disease, and other anterior segment disorders. She enjoys caring for and being a part of our community. She is on staff at Southampton Hospital and the Suffolk Surgery Center.
“Ophthalmology provides me ample opportunities to enrich the lives of my patients,” said Dr. Buono, “and I consider it an honor to help make a measurable difference in their lives every day.”
The entire medical staff at North Shore Eye Care is proud to have the husband and wife ophthalmic team on staff. “Dr. Buono and his wife have brought a wealth of knowledge in several areas of ophthalmic care to our practice and have been excellent additions to our growing team of surgeons,” said Dr. Martin.
The entire medical staff at North Shore Eye Care is proud to have the husband and wife ophthalmic team on staff. “Dr. Buono and his wife have brought a wealth of knowledge in several areas of ophthalmic care to our practice and have been excellent additions to our growing team of surgeons,” said Dr. Martin.
North Shore Eye Care is Long Island’s most established full-service comprehensive eye care provider. This year they are celebrating 50 years of eye care excellence since Dr. Sidney Martin founded the practice in 1962. North Shore Eye Care is also the official Eye Care Provider for the New York Islanders and the official LASIK Providers of the New York Mets. Many of their doctors have been voted ‘TOP DOCTORS’ in the New York Metro Area by Castle Connolly and North Shore Eye Care has earned ‘Best Of Long Island’ honors for the past few years.
North Shore Eye Care maintains offices in Smithtown, Riverhead, Holbrook, Deer Park, Southampton and Southold. They specialize in cataract care, LASIK laser vision correction, glaucoma management, diabetic eye disease, oculoplastics, neuro-ophthalmology, and retinal care. For more information about North Shore Eye Care, please contact Jacqueline Hernandez at 631-265-8780.
Contact Jacqueline Hernandez
Office: 631-265-8780
Email: Jacqueline(at)nsEYE(dot)com
Office: 631-265-8780
Email: Jacqueline(at)nsEYE(dot)com
From: Google Alerts <googlealerts-noreply@google.com>
Date: Fri, Sep 13, 2013 at 4:26 PM
Subject: Google Alert - neuro-ophthalmologists
To: atholiya@gmail.com
http://www.prweb.com/releases/2013/9/prweb11114115.htm
Date: Fri, Sep 13, 2013 at 4:26 PM
Subject: Google Alert - neuro-ophthalmologists
To: atholiya@gmail.com
http://www.prweb.com/releases/2013/9/prweb11114115.htm
| News | 1 new result for neuro-ophthalmologists |
| North Shore Eye Care Adds Ophthalmologists Lawrence Buono, MD ...PR Web (press release)
Upon completion of his residency, he completed a neuro-ophthalmology fellowship at the Wills Eye Hospital in Philadelphia. Dr. Buono then served as an ...
See all stories on this topic » | ||
Thursday, September 5, 2013
I am diagonised with Right Mastoiditis and Chronic Right Maxillary sinus.....Shows MRI @ Ambani : on 4ht Sep 2013
Do not know whether to go to ENT or Neurologist or Onco or Interventionist or ???? Pl. talk to ur friend doctor if any and advise......Alok
Why I went for MRI: I hv a Right optic nerve and chiasm atrophy means right eye optic nuritis. Besides I hv constant headache on right side.
Mastoiditis
From Wikipedia, the free encyclopedia
Jump to: navigation, search
| Mastoiditis | |
|---|---|
| Classification and external resources | |
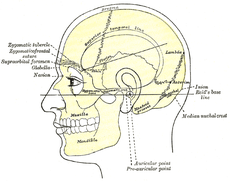
Side view of head, showing surface relations of bones. (Mastoid process labeled near center.)
|
|
| ICD-10 | H70 |
| ICD-9 | 383.0-383.1 |
| DiseasesDB | 22479 |
| MedlinePlus | 001034 |
| eMedicine | emerg/306 ped/1379 |
| MeSH | D008417 |
Contents
Features
Some common symptoms and signs of mastoiditis include pain, tenderness, and swelling in the mastoid region. There may be ear pain (otalgia), and the ear or mastoid region may be red (erythematous). Fever or headaches may also be present. Infants usually show nonspecific symptoms, including anorexia, diarrhea, or irritability. Drainage from the ear occurs in more serious cases, often manifest as brown discharge on the pillowcase upon waking.[4][5]Diagnosis
The diagnosis of mastoiditis is clinical—based on the medical history and physical examination. Imaging studies provide additional information; The standard method of diagnosis is via MRI scan although a CT scan is a common alternative as it gives a clearer and more useful image to see how close the damage may have gotten to the brain and facial nerves. Planar (2-D) X-rays are not as useful. If there is drainage, it is often sent for culture, although this will often be negative if the patient has begun taking antibiotics. Exploratory surgery is often used as a last resort method of diagnosis to see the mastoid and surrounding areas.[2][6]Pathophysiology
The pathophysiology of mastoiditis is straightforward: bacteria spread from the middle ear to the mastoid air cells, where the inflammation causes damage to the bony structures. Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, Haemophilus influenzae, and Moraxella catarrhalis are the most common organisms recovered in acute mastoiditis. Organisms that are rarely found are Pseudomonas aeruginosa and other Gram-negative aerobic bacilli, and anaerobic bacteria.[7] P. aeruginosa, Enterobacteriaceae, S. aureus and anaerobic bacteria (Prevotella, Bacteroides, Fusobacterium, and Peptostreptococcus spp. ) are the most common isolates in chronic mastoiditis.[8] Rarely, Mycobacterium species can also cause the infection. Some mastoiditis is caused by cholesteatoma, which is a sac of keratinizing squamous epithelium in the middle ear that usually results from repeated middle-ear infections. If left untreated, the cholesteatoma can erode into the mastoid process, producing mastoiditis, as well as other complications.[4]Prevention and treatment
In general, mastoiditis is rather simple to prevent. If the patient with an ear infection seeks treatment promptly and receives complete treatment, the antibiotics will usually cure the infection and prevent its spread. For this reason, mastoiditis is rare in developed countries. However, the rise of "superbugs" that are resistant to conventional antibiotics increases the risk that ear infections will worsen into mastoiditis. Most ear infections occur in infants as the eustachian tubes are not fully developed and don't drain readily.In the United States the primary treatment for mastoiditis is administration of intravenous antibiotics. Initially, broad-spectrum antibiotics are given, such as ceftriaxone. As culture results become available, treatment can be switched to more specific antibiotics directed at the eradication of the recovered aerobic and anaerobic bacteria.[8] Long-term antibiotics may be necessary to completely eradicate the infection.[4] If the condition does not quickly improve with antibiotics, surgical procedures may be performed (while continuing the medication). The most common procedure is a myringotomy, a small incision in the tympanic membrane (eardrum), or the insertion of a tympanostomy tube into the eardrum.[6] These serve to drain the pus from the middle ear, helping to treat the infection. The tube is extruded spontaneously after a few weeks to months, and the incision heals naturally. If there are complications, or the mastoiditis does not respond to the above treatments, it may be necessary to perform a mastoidectomy: a procedure in which a portion of the bone is removed and the infection drained.[4]
Prognosis
With prompt treatment, it is possible to cure mastoiditis. Seeking medical care early is important. However, it is difficult for antibiotics to penetrate to the interior of the mastoid process and so it may not be easy to cure the infection; it also may recur. Mastoiditis has many possible complications, all connected to the infection spreading to surrounding structures. Hearing loss is likely, or inflammation of the labyrinth of the inner ear (labyrinthitis) may occur, producing vertigo and an ear ringing may develop along with the hearing loss, making it more difficult to communicate. The infection may also spread to the facial nerve (cranial nerve VII), causing facial-nerve palsy, producing weakness or paralysis of some muscles of facial expression, on the same side of the face. Other complications include Bezold's abscess, an abscess (a collection of pus surrounded by inflamed tissue) behind the sternocleidomastoid muscle in the neck, or a subperiosteal abscess, between the periosteum and mastoid bone ( resulting in the typical appearance of a protruding ear). Serious complications result if the infection spreads to the brain. These include meningitis (inflammation of the protective membranes surrounding the brain), epidural abscess (abscess between the skull and outer membrane of the brain), dural venous thrombophlebitis (inflammation of the venous structures of the brain), or brain abscess.[2][4]Epidemiology
In the United States and other developed countries, the incidence of mastoiditis is quite low, around 0.004%, although it is higher in developing countries. The condition most commonly affects children aged from two to thirteen months, when ear infections most commonly occur. Males and females are equally affected.[3]References
- ^ Diseases of ear nose & throat by PL dhingra & shruti dhingra. published by elsevier
- ^ a b c d "Mastoiditis". MedlinePlus Medical Encyclopedia. Retrieved July 30, 2003.
- ^ a b "Ear Infections – Treatment". webmd.com. Retrieved 24 November 2008.
- ^ a b c d e f Young, Tesfa. "Mastoiditis". eMedicine. Retrieved June 10, 2005.
- ^ "What to Do About Ear infections". webmd.com. Retrieved 24 November 2008.
- ^ a b Bakhos D, Trijolet JP, Morinière S, Pondaven S, Al Zahrani M, Lescanne E (2011 Apr). "Conservative management of acute mastoiditis in children". Arch Otolaryngol Head Neck Surg. 137(4): 346–50.
- ^ Nussinovitch M, Yoeli R, Elishkevitz K, Varsano I (2004). "Acute mastoiditis in children: epidemiologic, clinical, microbiologic, and therapeutic aspects over past years". Clin Pediatr (Phila) 43: 261–7.
- ^ a b Brook I (2005). "The role of anaerobic bacteria in acute and chronic mastoiditis". Anaerobe 11: 252–7.
Further reading
- Durand, Marlene & Joseph, Michael. (2001). Infections of the Upper Respiratory Tract. In Eugene Braunwald, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, & J. Larry Jameson (Eds.), Harrison's Principles of Internal Medicine (15th Edition), p. 191. New York: McGraw-Hill
- Cummings CW, Flint PW, Haughey BH, et al. Otolaryngology: Head & Neck Surgery. 4th ed. St Louis, Mo; Mosby; 2005:3019–3020.
- Mastoiditis E Medicine
---------------------------------------------------------------------------------------------------------------
Maxillary sinus
From Wikipedia, the free encyclopedia
Jump to: navigation, search
| Maxillary sinus | |
|---|---|
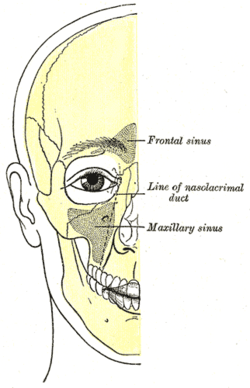 |
|
| Outline of bones of face, showing position of air sinuses. | |
| Latin | sinus maxilliaris |
| Gray's | subject #223 999 |
| Artery | infraorbital artery, posterior superior alveolar artery |
| Nerve | posterior superior alveolar nerve, middle superior alveolar nerve, anterior superior alveolar nerve, and infraorbital nerve |
| MeSH | Maxillary+Sinus |
Contents
Development
It is present at birth as rudimentary air cells, and develops throughout childhood.Discovery
The maxillary sinus was first discovered and illustrated by Leonardo da Vinci, but the earliest attribution of significance was given to Nathaniel Highmore, the British surgeon and anatomist who described it in detail in his 1651 treatise.[2]Structure
Found in the body of the maxilla, this sinus has three recesses: an alveolar recess pointed inferiorly, bounded by the alveolar process of the maxilla; a zygomatic recess pointed laterally, bounded by the zygomatic bone; and an infraorbital recess pointed superiorly, bounded by the inferior orbital surface of the maxilla. The medial wall is composed primarily of cartilage. The ostia for drainage are located high on the medial wall and open into the semilunar hiatus of the lateral nasal cavity; because of the position of the ostia, gravity cannot drain the maxillary sinus contents when the head is erect (see pathology). The ostium of the maxillary sinus is high up on the medial wall and on average is 2.4 mm in diameter; with a mean volume of about 10 ml.[3][1] Stand near the person during an extraoral examination to visually inspect and bilaterally palpate the maxillary sinuses.[4]The sinus is lined with mucoperiosteum, with cilia that beat toward the ostia. This membrane is also referred to as the "Schneiderian Membrane", which is histologically a bilaminar membrane with ciliated columnar epithelial cells on the internal (or cavernous) side and periosteum on the osseous side. The size of the sinuses varies in different skulls, and even on the two sides of the same skull.[3]
The infraorbital canal usually projects into the cavity as a well-marked ridge extending from the roof to the anterior wall; additional ridges are sometimes seen in the posterior wall of the cavity and are caused by the alveolar canals.
The mucous membranes receive their postganglionic parasympathetic nerve innervation for mucous secretion originating from the greater petrosal nerve (a branch of the facial nerve). The superior alveolar (anterior, middle, and posterior) nerves, branches of the maxillary nerve provide sensory innervation.
Nasal wall/base
Its nasal wall, or base, presents, in the disarticulated bone, a large, irregular aperture, communicating with the nasal cavity.In the articulated skull this aperture is much reduced in size by the following bones:
- the uncinate process of the ethmoid above,
- the ethmoidal process of the inferior nasal concha below,
- the vertical part of the palatine behind,
- and a small part of the lacrimal above and in front.
Posterior wall
On the posterior wall are the alveolar canals, transmitting the posterior superior alveolar vessels and nerves to the molar teeth.Floor

The maxillary sinus can normally be seen above the level of the premolar and molar teeth in the upper jaw. This dental x-ray film shows how, in the absence of the second premolar and first molar, the sinus became pneumatized and expanded towards the crest of the alveolar process (location at which the bone meets the gum tissue).
Projecting into the floor of the antrum are several conical processes, corresponding to the roots of the first and second maxillary molar teeth; in some cases the floor can be perforated by the apices of the teeth.
Pathology
Maxillary sinusitis
Maxillary sinusitis is inflammation of the maxillary sinuses. The symptoms of sinusitis are headache, usually near the involved sinus, and foul-smelling nasal or pharyngeal discharge, possibly with some systemic signs of infection such as fever and weakness. The skin over the involved sinus can be tender, hot, and even reddened due to the inflammatory process in the area. On radiographs, there is opacification (or cloudiness) of the usually translucent sinus due to retained mucus.[4]Maxillary sinusitis is common due to the close anatomic relation of the frontal sinus, anterior ethmoidal sinus and the maxillary teeth, allowing for easy spread of infection. Differential diagnosis of dental problems needs to be done due to the close proximity to the teeth since the pain from sinusitis can seem to be dentally related.[1] Furthermore, the drainage orifice lies near the roof of the sinus, and so the maxillary sinus does not drain well, and infection develops more easily. The maxillary sinus may drain into the mouth via an abnormal opening, an oroantral fistula, a particular risk after tooth extraction.
Sinusitis treatment
Traditionally the treatment of acute maxillary sinusitis is usually prescription of a broad-spectrum cephalosporin antibiotic resistant to beta-lactamase, administered for 10 days. Recent studies have found that the cause of chronic sinus infections lies in the nasal mucus, not in the nasal and sinus tissue targeted by standard treatment. This suggests a beneficial effect in treatments that target primarily the underlying and presumably damage-inflicting nasal and sinus membrane inflammation, instead of the secondary bacterial infection that has been the primary target of past treatments for the disease. Also, surgical procedures with chronic sinus infections are now changing with the direct removal of the mucus, which is loaded with toxins from the inflammatory cells, rather than the inflamed tissue during surgery. Leaving the mucus behind might predispose early recurrence of the chronic sinus infection. If any surgery is performed, it is to enlarge the ostia in the lateral walls of the nasal cavity, creating adequate drainage.[4]Cancer
Carcinoma of the maxillary sinus may invade the palate and cause dental pain. It may also block the nasolacrimal duct. Spread of the tumor into the orbit causes proptosis.[1]Age
With age, the enlarging maxillary sinus may even begin to surround the roots of the maxillary posterior teeth and extend its margins into the body of the zygomatic bone. If the maxillary posterior teeth are lost, the maxillary sinus may expand even more, thinning the bony floor of the alveolar process so that only a thin shell of bone is present.[4]Additional Images
Mastoiditis
Mastoiditis is an infection of the mastoid bone of the skull. The mastoid is located just behind the ear.
Causes
Mastoiditis is usually caused by a middle ear infection (acute otitis media). The infection may spread from the ear to the mastoid bone of the skull. The mastoid bone fills with infected materials and its honeycomb-like structure may deteriorate.Mastoiditis usually affects children. Before antibiotics, mastoiditis was one of the leading causes of death in children. Now it is a relatively uncommon and much less dangerous condition.
Symptoms
- Drainage from the ear
- Ear pain or discomfort
- Fever, may be high or suddenly increase
- Headache
- Hearing loss
- Redness of the ear or behind the ear
- Swelling behind ear, may cause ear to stick out
Exams and Tests
An examination of the head may reveal signs of mastoiditis. The following tests may show an abnormality of the mastoid bone:- CTscan of the ear
- Head CT scan
Treatment
Mastoiditis may be difficult to treat because medications may not reach deep enough into the mastoid bone. It may require repeated or long-term treatment. The infection is treated with antibiotics by injection, then antibiotics by mouth.Surgery to remove part of the bone and drain the mastoid (mastoidectomy) may be needed if antibiotic therapy is not successful. Surgery to drain the middle ear through the eardrum (myringotomy) may be needed to treat the middle ear infection.
Outlook (Prognosis)
Mastoiditis is curable with treatment. However, it may be hard to treat and may come back.Possible Complications
- Destruction of the mastoid bone
- Dizziness or vertigo
- Epidural abscess
- Facial paralysis
- Meningitis
- Partial or complete hearing loss
- Spread of infection to the brain or throughout the body
When to Contact a Medical Professional
Call your health care provider if you have symptoms of mastoiditis.Call for an appointment with your health care provider if:
- You have an ear infection that does not respond to treatment or is followed by new symptoms
- Your symptoms do not respond to treatment
Prevention
Promptly and completely treating ear infections reduces the risk of mastoiditis.References
Chole RA, Sudhoff HH. Chronic otitis media, mastoiditis, and petrositis. In: Flint PW, Haughey BH, Lund LJ, et al, eds. Cummings Otolaryngology: Head & Neck Surgery. 5th ed. Philadelphia, Pa: Mosby Elsevier; 2010:chap 139.Klein JO. Otitis externa, otitis media, and mastoiditis. In:Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009:chap57.
O’Handley JG, Tobin EJ, Shah AR. Otorhinolaryngology. In: Rakel RE, ed. Textbook of Family Medicine. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 19.
Update Date: 8/30/2012
Updated by: Linda J. Vorvick, MD, Medical Director and Director of Didactic Curriculum, MEDEX Northwest Division of Physician Assistant Studies, Department of Family Medicine, UW Medicine, School of Medicine, University of Washington. Seth Schwartz, MD, MPH, Otolaryngologist, Virginia Mason Medical Center, Seattle, Washington. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M. Health Solutions, Ebix, IncWednesday, May 1, 2013
Dr Vaibhav 's advise to trat thru Ayurved
|
Jan 23
 |   | ||
| ||||
Personal corresspondence on right eye
|
Feb 25
 |   | ||
| ||||
Bottom Line on Top: Please keep a copy of all your medical records and doctors opinions ready for me.
I am very excited to see you all soon next week and meet you.
In this short trip with one of the big events in my life, I know
time is going to fly by. I realize in the rush and hurry might not be
able to even greet everyone with as much time as I would personally
want. But in the midst of all this, I do not want to miss the
opportunity to get a copy (digital or photocopy) of all your records
regarding the Glaucoma.
I want to talk to some specialists here and get a second opinion. I don't see any harm in trying.
I
do not have names of any particular doctors in mind, but I will surely
do my research and consult a few of them. So if you know about
some hospital or doctor that are good, you can include those details too
in the records copy.
I am waiting to see you and get your blessings before the big day in my life.
Love you
Your mastikhor bhanja
Pampoo
On Mon, Jan 28, 2013 at 6:56 AM, Alok Tholiya <atholiya@gmail.com> wrote:
As usual my self own my own
found that there is some development in my right eye. No one believed me
initially. Even eye specialist did not diagonise nor first believed
what I was saying as I am just 58. Then he must have realized I may have
Glaucoma.Doc then sent me for tests .
Great news. 75% vision in my right is permanently lost.
These are messages from almighty that has started liking me and
considering my name for his invite list and Yamdut is busy so unless it
is urgent it may take few years for him to send me invite.
In the meanwhile he has given me time to :
1. Stretegise my plans for retirement
2. enjoy more time with meditation and keep in touch with him
3.
spend some time on health so I am less burden to active members in
family ( but I hv to develop will power as knowing that is not enough)
4. Given me alert to Beautifully design my will and last testament.
5.
Increase pace of good work for humanity ( next item on agenda is
Ambulance for society).( my novel idea Day creche / Palna Ghar for Dada
Dadi ( Sr. citizens ) so earning members can attend to office/
emergency seems little distant)
6. Tour some destinations longed for whole life that one day I will be
free and will be able to find time. Finally I thought post my dear
daughters marriage I will be free when I hand over her to a loving ,
caring , honest , character worthy husband and his family but .....but
if wishes were horses fools would ride. As bad-luck was chasing me we
tied know to Nishant Paras Jain, who had connived with Vidhi and Shilpa
to fool and cheat us so I further became busy and tormented. Police,
Courts, fraud stree Chetana and its advocate Helen and others are
further delaying the matter and siding with wife cheater and beater
Nishant Jain by delaying the justice. Insha Allah things will be alright
as I have not learnt to bow down to wrongdoers and will ultimately come
out victorious as it is rightly said that there is delay but not
injustice in the darbar of Almighty.
7. I will now be able to raise more vehemently issues dear to me ,
fight for right, RTI, activism, fight police and courts who have
different procedures and standards for different set of people and so
on.
Hope you will enjoy , celebrate and
become enlightened with this news of my loss of 75% vision and take it
as a step reaching near and dear GOD and will not waste time on asking
several questions on this development.
Why I am making this open as I am not the one who tries to keep
everything secret and hidden. Read me like a open book. ...........

Tuesday, April 30, 2013
How a laser could prevent age-related blindness
How a laser could prevent age-related blindness
By Pat HaganPUBLISHED:21:18 GMT, 29 April 2013| UPDATED:06:40 GMT, 30 April 2013
The laser works by firing pulses that take only three nanoseconds — three billionths of a second — to hit their target at the back of the eye.
These destroy deposits that can lead to age-related macular degeneration (AMD), the UK’s leading cause of blindness and a condition that affects around 600,000 people. With Britain’s ageing population, the numbers left blind by it are set to rise.
The laser works by firing pulses that take only three nanoseconds - three billionths of a second - to hit their target at the back of the eye
AMD usually develops after the age of 50 and is caused by the growth of new blood vessels over the macula, an oval-shaped area at the back of the eye that helps us pick out visual details clearly.
These blood vessels leak fluid, causing scar tissue to form and destroy vision in the centre of the eye, making it difficult to recognise faces, read or watch TV.
The rest involve wet AMD, which can cause blindness in as little as three months. Treatment involves monthly injections into the back of the eye with drugs designed to curb the growth of abnormal blood vessels. Administered early, it can prevent complete loss of vision but cannot reverse the disease.
But scientists hope the new laser therapy will be able to halt both forms of AMD in their tracks before any vision is lost. This is because before abnormal blood vessels start to grow, yellow-white deposits called drusen often form at the back of the eye. These are made up of lipids, a type of fat, and accumulate when the ageing eye becomes less efficient at disposing of waste from cells that have died off naturally.
Small, widely scattered deposits are not normally a problem. But large ones that are closer together can trigger the process that leads to the growth of abnormal blood vessels because new vessels form to bypass the harmful deposits.
Previous laser therapies have had limited success and can cause collateral damage to the surrounding healthy eye tissue — despite lasting a few hundredths of a second, their pulses still generate enough heat to harm healthy areas.
But the nanosecond laser, the Ellex 2RT, is so fast and accurate it appears to leave this tissue unscathed.
Usually, the patient needs only 12 pulses per eye, so total treatment time is less than half a second.

Previous laser therapies have had limited success and can cause collateral damage to the surrounding healthy eye tissue
Professor Robyn Guymer, who led the study at the Centre for Eye Research Australia in Melbourne, said: ‘Patients reported that the treatment was completely painless. By getting rid of the fatty deposits, we hope to reverse the degenerative processes caused by the disease.’
Dr Sobha Sivaprasad, consultant ophthalmologist at King’s College Hospital in London, said the therapy might prove helpful if larger-scale trials show it actually reduces the incidence of AMD.
But she stressed that fatty deposits in the eye are thought to be only one of several risk factors for AMD. Others include obesity, heart disease and family history.
Manwhile, patients with age-related macular degeneration are being given a dose of the impotence drug Viagra in a clinical trial at Duke University in the U.S.
It is thought to boost the choroid, the layer of tissue that covers the retina (the light-sensitive tissue at the back of the eye).
With age, the choroid can become thinner, leading to the formation of drusen.
One theory is that thinning of the choroid is involved in the development of AMD — so thickening it could potentially slow down the course of the disease.
Earlier studies have shown that a single dose of sildenafil citrate, or Viagra, appears to thicken the choroid in young healthy patients.
Read more: http://www.dailymail.co.uk/health/article-2316800/How-laser-prevent-age-related-blindness.html#ixzz2RzZjqntx
Follow us: @MailOnline on Twitter | DailyMail on Facebook
Monday, April 29, 2013
Thursday, April 25, 2013
Monday, April 22, 2013
Advise from Dr P K Goswami USA ....Vipresh
Dear Vipresh
I have received your mail. I am sorry that I am late in
replying. I have studied the case history and all the investigations inserted in the mail
regarding the eye condition of your
uncle (Mamaji ) . Based on those findings, it appears to me that your uncle may
be suffering from “Optic neuritis”, a
neuro-ophthalmic condition, rather than Glaucoma. MRI findings and
visually evoked potential (VEP ) indicate optic neuritis. Though his Humphrey auto
perimetry report is not classical of
optic neuritis, the field may change during the course of the disease. I am not
sure about the optic disc changes which are very essential to diagnose a case
of glaucoma. In this situation , further
tests like OCT and Heidelberg retinal tomography by an ophthalmologist
are needed to thoroughly evaluate the
optic disc of the eyes. Optic neuritis
may be due to different causes
which the Neuro-ophthalmologist will
evaluate and give treatment according to the course of the
disease. There is a rare possibility of a condition called meningioma of
the optic nerve sheath of right eye, a rare
benign tumour which should be excluded by critical evaluation of the MRI. I don’t know whether he has regained some amount of his vision now. I suggest
him to attend the glaucoma facility of Dr R. P Centre of Ophthalmic Science,
AIIMS, New Delhi if possible.
Thanking you for refer ring to me and wish your uncle to recover
soon.
Dr P.K.Goswami
Friday, April 19, 2013
Home » Useful Resources for EYE
| ||||||||||||||||
Heidelberg Retina Tomograph (HRT)
Heidelberg Retina Tomograph (HRT)
Most people equate the eye disease called glaucoma with high eye pressure. It is important to understand that glaucoma is not simply a disease of high intra-ocular pressure, but rather, a more complex disease of the optic nerve. In the early stages of glaucoma there are no symptoms and understandably any condition that lacks symptoms has great potential risk. In glaucoma the vision conductive rim of the optic nerve, called the nerve-fiber layer, is gradually damaged resulting in a lessening of visual information leaving the eye to the brain. We now understand that measurement of eye pressure is only one small part of the data collected for doctors to evaluate the total picture in regard to glaucoma. Up until now the tests utilized to diagnose glaucoma and establish the need for treatment was based on eye pressure and documented vision loss and a diagnosis was only possible after vision loss had already taken place.
Recent developments in computer imaging technology now allow us to image the sensitive nerve-fiber layer of the optic nerve utilizing a three-dimensional cross-section view. The Heidelberg Retinal Tomograph (HRT) is a system that combines a laser-scanning camera and specialized software that evaluates the optic nerve. For the first time this revolutionary technology allows us to understand the progression of optic nerve involvement in regard to glaucoma and other eye conditions long before irreversible vision loss takes place. Tomography utilizes real-time information of the living eye for immediate study and analysis. In addition, each captured image is compared utilizing a normal outcomes database of a patient's age. It has been shown that 3-D measurements of the optic nerve head are far superior to conventional examination methods.
The HRT works something like an ultrasound, but rather than sound, the process utilizes reflected light and converts the layered image into an enhanced color image. The HRT exam takes just a few minutes and it is a painless non-invasive test. Usually dilation of the eye is not necessary. This technology allows us to more accurately follow disease progression and treatment options and has now become the standard of care for patients with documented cautions, and/or, a strong family history of glaucoma. The key to controlling glaucoma is catching it early. The best way to prevent vision loss from glaucoma is to know your risk factors and to have an eye examination at appropriate intervals.
Do you have OCT tests facility at your eye clinic / hospital
Dr P K Goswami from USA has advised me to go for OCT test. Pl. advise where in Mumbai/ India same is done?
Optical coherence tomography
From Wikipedia, the free encyclopedia
| Optical coherence tomography | |
|---|---|
| Intervention | |
 Optical Coherence Tomography (OCT) image of a sarcoma | |
| MeSH | D041623 |
| OPS-301 code: | 3-300 |
Optical coherence tomography (OCT) is an optical signal acquisition and processing method. It captures micrometer-resolution, three-dimensional images from within optical scattering media (e.g., biological tissue). Optical coherence tomography is aninterferometric technique, typically employing near-infrared light. The use of relatively longwavelength light allows it to penetrate into the scattering medium. Confocal microscopy, another similar technique, typically penetrates less deeply into the sample.
Depending on the properties of the light source (superluminescent diodes, ultrashort pulsed lasers and supercontinuum lasers have been employed), optical coherence tomography has achieved sub-micrometer resolution (with very wide-spectrum sources emitting over a ~100 nm wavelength range).
Optical coherence tomography is one of a class of optical tomographic techniques. A relatively recent implementation of optical coherence tomography, frequency-domainoptical coherence tomography, provides advantages in signal-to-noise ratio, permitting faster signal acquisition. Commercially available optical coherence tomography systems are employed in diverse applications, including art conservation and diagnostic medicine, notably in ophthalmology where it can be used to obtain detailed images from within the retina. Recently it has also begun to be used in interventional cardiology to help diagnose coronary artery disease.[1]
Contents[hide] |
[edit]Introduction
Starting from white-light interferometry for in vivo ocular eye measurements[2][3] imaging of biological tissue, especially of the human eye, was investigated by multiple groups worldwide. A first two-dimensional in vivo depiction of a human eye fundus along a horizontal meridian based on white light interferometric depth scans was presented at the ICO-15 SAT conference in 1990.[4] Further developed in 1990 by Naohiro Tanno,[5][6] then a professor at Yamagata University, and in particular since 1991 by Huang et al.,[7] optical coherence tomography (OCT) with micrometer resolution and cross-sectional imaging capabilities has become a prominent biomedical tissue-imaging technique; it is particularly suited to ophthalmic applications and other tissue imaging requiring micrometer resolution and millimeter penetration depth.[8] First in vivo OCT images – displaying retinal structures – were published in 1993.[9][10] OCT has also been used for various art conservation projects, where it is used to analyze different layers in a painting. OCT has critical advantages over other medical imagingsystems. Medical ultrasonography, magnetic resonance imaging (MRI) and confocal microscopy are not suited to morphological tissue imaging: the first two have poor resolution; the last lacks millimeter penetration depth.[11][12]
OCT bases itself upon low coherence interferometry.[13][14][15] In conventional interferometry with long coherence length (laser interferometry), interference of light occurs over a distance of meters. In OCT, this interference is shortened to a distance of micrometers, thanks to the use of broadband light sources (sources that can emit light over a broad range of frequencies). Light with broad bandwidths can be generated by using superluminescent diodes (superbright LEDs) or lasers with extremely short pulses (femtosecond lasers). White light is also a broadband source with lower power.
Light in an OCT system is broken into two arms—a sample arm (containing the item of interest) and a reference arm (usually a mirror). The combination of reflected light from the sample arm and reference light from the reference arm gives rise to an interference pattern, but only if light from both arms have travelled the "same" optical distance ("same" meaning a difference of less than a coherence length). By scanning the mirror in the reference arm, a reflectivity profile of the sample can be obtained (this is time domain OCT). Areas of the sample that reflect back a lot of light will create greater interference than areas that don't. Any light that is outside the short coherence length will not interfere. This reflectivity profile, called an A-scan, contains information about the spatial dimensions and location of structures within the item of interest. A cross-sectional tomograph (B-scan) may be achieved by laterally combining a series of these axial depth scans (A-scan). En face imaging at an acquired depth is possible depending on the imaging engine used.
[edit]Layperson's explanation
Optical Coherence Tomography, or ‘OCT’, is a technique for obtaining sub-surface images of translucent or opaque materials at a resolution equivalent to a low-power microscope. It is effectively ‘optical ultrasound’, imaging reflections from within tissue to provide cross-sectional images.
OCT is attracting interest among the medical community, because it provides tissue morphology imagery at much higher resolution (better than 10 µm) than other imaging modalities such as MRI or ultrasound.
The key benefits of OCT are:
- Live sub-surface images at near-microscopic resolution
- Instant, direct imaging of tissue morphology
- No preparation of the sample or subject
- No ionizing radiation
OCT delivers high resolution because it is based on light, rather than sound or radio frequency. An optical beam is directed at the tissue, and a small portion of this light that reflects from sub-surface features is collected. Note that most light is not reflected but, rather, scatters off at large angles. In conventional imaging, this diffusely scattered light contributes background that obscures an image. However, in OCT, a technique called interferometry is used to record the optical path length of received photons allowing rejection of most photons that scatter multiple times before detection. Thus OCT can build up clear 3D images of thick samples by rejecting background signal while collecting light directly reflected from surfaces of interest.
Within the range of noninvasive three-dimensional imaging techniques that have been introduced to the medical research community, OCT as an echo technique is similar to ultrasound imaging. Other medical imaging techniques such as computerized axial tomography, magnetic resonance imaging, or positron emission tomography do not utilize the echo-location principle.
The technique is limited to imaging 1 to 2 mm below the surface in biological tissue, because at greater depths the proportion of light that escapes without scattering is too small to be detected. No special preparation of a biological specimen is required, and images can be obtained ‘non-contact’ or through a transparent window or membrane. It is also important to note that the laser output from the instruments is low – eye-safe near-infra-red light is used – and no damage to the sample is therefore likely.
[edit]Theory
The principle OCT is white light or low coherence interferometry. The optical setup typically consists of an interferometer (Fig. 1, typically Michelson type) with a low coherence, broad bandwidth light source. Light is split into and recombined from reference and sample arm, respectively.
[edit]Time domain OCT
In time domain OCT the pathlength of the reference arm is translated longitudinally in time. A property of low coherence interferometry is that interference, i.e. the series of dark and bright fringes, is only achieved when the path difference lies within the coherence length of the light source. This interference is called auto correlation in a symmetric interferometer (both arms have the same reflectivity), or cross-correlation in the common case. The envelope of this modulation changes as pathlength difference is varied, where the peak of the envelope corresponds to pathlength matching.
The interference of two partially coherent light beams can be expressed in terms of the source intensity,  , as
, as
 , as
, as
where  represents the interferometer beam splitting ratio, and
represents the interferometer beam splitting ratio, and  is called the complex degree of coherence, i.e. the interference envelope and carrier dependent on reference arm scan or time delay
is called the complex degree of coherence, i.e. the interference envelope and carrier dependent on reference arm scan or time delay  , and whose recovery of interest in OCT. Due to the coherence gating effect of OCT the complex degree of coherence is represented as a Gaussian function expressed as[15]
, and whose recovery of interest in OCT. Due to the coherence gating effect of OCT the complex degree of coherence is represented as a Gaussian function expressed as[15]
 represents the interferometer beam splitting ratio, and
represents the interferometer beam splitting ratio, and  is called the complex degree of coherence, i.e. the interference envelope and carrier dependent on reference arm scan or time delay
is called the complex degree of coherence, i.e. the interference envelope and carrier dependent on reference arm scan or time delay  , and whose recovery of interest in OCT. Due to the coherence gating effect of OCT the complex degree of coherence is represented as a Gaussian function expressed as[15]
, and whose recovery of interest in OCT. Due to the coherence gating effect of OCT the complex degree of coherence is represented as a Gaussian function expressed as[15]
where  represents the spectral width of the source in the optical frequency domain, and
represents the spectral width of the source in the optical frequency domain, and  is the centre optical frequency of the source. In equation (2), the Gaussian envelope is amplitude modulated by an optical carrier. The peak of this envelope represents the location of sample under test microstructure, with an amplitude dependent on the reflectivity of the surface. The optical carrier is due to the Doppler effect resulting from scanning one arm of the interferometer, and the frequency of this modulation is controlled by the speed of scanning. Therefore translating one arm of the interferometer has two functions; depth scanning and a Doppler-shifted optical carrier are accomplished by pathlength variation. In OCT, the Doppler-shifted optical carrier has a frequency expressed as
is the centre optical frequency of the source. In equation (2), the Gaussian envelope is amplitude modulated by an optical carrier. The peak of this envelope represents the location of sample under test microstructure, with an amplitude dependent on the reflectivity of the surface. The optical carrier is due to the Doppler effect resulting from scanning one arm of the interferometer, and the frequency of this modulation is controlled by the speed of scanning. Therefore translating one arm of the interferometer has two functions; depth scanning and a Doppler-shifted optical carrier are accomplished by pathlength variation. In OCT, the Doppler-shifted optical carrier has a frequency expressed as
 represents the spectral width of the source in the optical frequency domain, and
represents the spectral width of the source in the optical frequency domain, and  is the centre optical frequency of the source. In equation (2), the Gaussian envelope is amplitude modulated by an optical carrier. The peak of this envelope represents the location of sample under test microstructure, with an amplitude dependent on the reflectivity of the surface. The optical carrier is due to the Doppler effect resulting from scanning one arm of the interferometer, and the frequency of this modulation is controlled by the speed of scanning. Therefore translating one arm of the interferometer has two functions; depth scanning and a Doppler-shifted optical carrier are accomplished by pathlength variation. In OCT, the Doppler-shifted optical carrier has a frequency expressed as
is the centre optical frequency of the source. In equation (2), the Gaussian envelope is amplitude modulated by an optical carrier. The peak of this envelope represents the location of sample under test microstructure, with an amplitude dependent on the reflectivity of the surface. The optical carrier is due to the Doppler effect resulting from scanning one arm of the interferometer, and the frequency of this modulation is controlled by the speed of scanning. Therefore translating one arm of the interferometer has two functions; depth scanning and a Doppler-shifted optical carrier are accomplished by pathlength variation. In OCT, the Doppler-shifted optical carrier has a frequency expressed as
where  is the central optical frequency of the source,
is the central optical frequency of the source,  is the scanning velocity of the pathlength variation, and
is the scanning velocity of the pathlength variation, and  is the speed of light.
is the speed of light.
 is the central optical frequency of the source,
is the central optical frequency of the source,  is the scanning velocity of the pathlength variation, and
is the scanning velocity of the pathlength variation, and  is the speed of light.
is the speed of light.
The axial and lateral resolutions of OCT are decoupled from one another; the former being an equivalent to the coherence length of the light source and the latter being a function of the optics. The coherence length of a source and hence the axial resolution of OCT is defined as
[edit]Frequency domain OCT (FD-OCT)
In frequency domain OCT the broadband interference is acquired with spectrally separated detectors (either by encoding the optical frequency in time with a spectrally scanning source or with a dispersive detector, like a grating and a linear detector array). Due to theFourier relation (Wiener-Khintchine theorem between the auto correlation and the spectral power density) the depth scan can be immediately calculated by a Fourier-transform from the acquired spectra, without movement of the reference arm.[16][17] This feature improves imaging speed dramatically, while the reduced losses during a single scan improve the signal to noise proportional to the number of detection elements. The parallel detection at multiple wavelength ranges limits the scanning range, while the full spectral bandwidth sets the axial resolution.
[edit]Spatially encoded frequency domain OCT (spectral domain or Fourier domain OCT)
SEFD-OCT extracts spectral information by distributing different optical frequencies onto a detector stripe (line-array CCD or CMOS) via a dispersive element (see Fig. 4). Thereby the information of the full depth scan can be acquired within a single exposure. However, the large signal to noise advantage of FD-OCT is reduced due the lower dynamic range of stripe detectors in respect to single photosensitive diodes, resulting in an SNR (signal to noise ratio) advantage of ~10 dB at much higher speeds. This is not much of a problem when working at 1300 nm, however, since dynamic range is not a serious problem at this wavelength range.
The drawbacks of this technology are found in a strong fall-off of the SNR, which is proportional to the distance from the zero delay and a sinc-type reduction of the depth dependent sensitivity because of limited detection linewidth. (One pixel detects a quasi-rectangular portion of an optical frequency range instead of a single frequency, the Fourier-transform leads to the sinc(z) behavior). Additionally the dispersive elements in the spectroscopic detector usually do not distribute the light equally spaced in frequency on the detector, but mostly have an inverse dependence. Therefore the signal has to be resampled before processing, which can not take care of the difference in local (pixelwise) bandwidth, which results in further reduction of the signal quality. However, the fall-off is not a serious problem with the development of new generation CCD or photodiode array with a larger number of pixels.
Synthetic array heterodyne detection offers another approach to this problem without the need for high dispersion.
[edit]Time encoded frequency domain OCT (also swept source OCT)
TEFD-OCT tries to combine some of the advantages of standard TD and SEFD-OCT. Here the spectral components are not encoded by spatial separation, but they are encoded in time. The spectrum either filtered or generated in single successive frequency steps and reconstructed before Fourier-transformation. By accommodation of a frequency scanning light source (i.e. frequency scanning laser) the optical setup (see Fig. 5) becomes simpler than SEFD, but the problem of scanning is essentially translated from the TD-OCT reference-arm into the TEFD-OCT light source. Here the advantage lies in the proven high SNR detection technology, while swept laser sources achieve very small instantaneous bandwidths (=linewidth) at very high frequencies (20–200 kHz). Drawbacks are the nonlinearities in the wavelength (especially at high scanning frequencies), the broadening of the linewidth at high frequencies and a high sensitivity to movements of the scanning geometry or the sample (below the range of nanometers within successive frequency steps).
[edit]Scanning schemes
Focusing the light beam to a point on the surface of the sample under test, and recombining the reflected light with the reference will yield an interferogram with sample information corresponding to a single A-scan (Z axis only). Scanning of the sample can be accomplished by either scanning the light on the sample, or by moving the sample under test. A linear scan will yield a two-dimensional data set corresponding to a cross-sectional image (X-Z axes scan), whereas an area scan achieves a three-dimensional data set corresponding to a volumetric image (X-Y-Z axes scan), also called full-field OCT.
[edit]Single point (confocal) OCT
Systems based on single point, or flying-spot time domain OCT, must scan the sample in two lateral dimensions and reconstruct a three-dimensional image using depth information obtained by coherence-gating through an axially scanning reference arm (Fig. 2). Two-dimensional lateral scanning has been electromechanically implemented by moving the sample[17] using a translation stage, and using a novel micro-electro-mechanical system scanner.[18]
[edit]Parallel (or full field) OCT
Parallel OCT using a charge-coupled device (CCD) camera has been used in which the sample is full-field illuminated and en face imaged with the CCD, hence eliminating the electromechanical lateral scan. By stepping the reference mirror and recording successiveen face images a three-dimensional representation can be reconstructed. Three-dimensional OCT using a CCD camera was demonstrated in a phase-stepped technique,[19] using geometric phase-shifting with a Linnik interferometer,[20] utilising a pair of CCDs and heterodyne detection,[21] and in a Linnik interferometer with an oscillating reference mirror and axial translation stage.[22] Central to the CCD approach is the necessity for either very fast CCDs or carrier generation separate to the stepping reference mirror to track the high frequency OCT carrier.
[edit]Smart detector array for parallel TD-OCT
A two-dimensional smart detector array, fabricated using a 2 µm complementary metal-oxide-semiconductor (CMOS) process, was used to demonstrate full-field OCT.[23] Featuring an uncomplicated optical setup (Fig. 3), each pixel of the 58x58 pixel smart detector array acted as an individual photodiode and included its own hardware demodulation circuitry.
[edit]Selected applications
Optical coherence tomography is an established medical imaging technique. It is widely used, for example, to obtain high-resolution images of the anterior segment of the eye and the retina, which can, for example, provide a straightforward method of assessing axonal integrity in multiple sclerosis,[24] as well as macular degeneration.[25] Research indicates that OCT may be a reliable tool for monitoring the progression of glaucoma. Researchers also seek to develop a method that uses frequency domain OCT to image coronary arteriesin order to detect vulnerable lipid-rich plaques.
Optical coherence tomography is also applicable and increasingly used in industrial applications, such as Non Destructive Testing(NDT), material thickness measurements,[26] and in particular thin silicon wafers[27],[28] and compound semiconductor wafers thickness measurements[29],,[30] surface roughness characterization, surface and cross-section imaging[31],,[32] and volume loss measurements. OCT systems with feedback can be used to control manufacturing processes. With high speed data acquisition,[33]and sub-micron resolution, OCT is adaptable to perform both inline and off-line.[34] Fiber-based OCT systems are particularly adaptable to industrial environments.[35] These can access and scan interiors of hard-to-reach spaces,[36] and are able to operate in hostile environments - whether radioactive, cryogenic or very hot.[37]
[edit]See also
- Interferometry
- Tomography
- Angle-resolved low-coherence interferometry
- Ballistic photon
- Optical heterodyne detection
- Novacam Technologies
OFDI is used to image the plaques in the artery based on bifringence property of the tissues.
[edit]References
- ^ Bezerra, Hiram G.; Costa, Marco A.; Guagliumi, Giulio; Rollins, Andrew M.; Simon, Daniel I. (November 2009). "Intracoronary Optical Coherence Tomography: A Comprehensive Review".JACC: Cardiovascular Interventions 2 (11): 1035–1046.doi:10.1016/j.jcin.2009.06.019. PMID 19926041.
- ^ A. F. Fercher and E. Roth, "Ophthalmic laser interferometry. Proc. SPIE vol. 658, pp. 48-51. 1986.
- ^ Fercher, AF; Mengedoht, K; Werner, W (1988). "Eye-length measurement by interferometry with partially coherent light.".Optics letters 13 (3): 186–8. Bibcode:1988OptL...13..186F.doi:10.1364/OL.13.000186. PMID 19742022.
- ^ A. F. Fercher, "Ophthalmic interferometry," Proceedings of the International Conference on Optics in Life Sciences, Garmisch-Partenkirchen, Germany, 12–16 August 1990. Ed. G. von Bally and S. Khanna, pp. 221-228. ISBN 0-444-89860-3.
- ^ Naohiro Tanno, Tsutomu Ichikawa, Akio Saeki: "Lightwave Reflection Measurement," Japanese Patent # 2010042 (1990) (Japanese Language)
- ^ Shinji Chiba, Naohiro Tanno "Backscattering Optical Heterodyne Tomography", prepared for the 14th Laser Sensing Symposium (1991) (in Japanese)
- ^ Huang, D; Swanson, EA; Lin, CP; Schuman, JS; Stinson, WG; Chang, W; Hee, MR; Flotte, T et al. (1991). "Optical coherence tomography.". Science 254 (5035): 1178–81.Bibcode:1991Sci...254.1178H.doi:10.1126/science.1957169. PMID 1957169.
- ^ Zysk, AM; Nguyen, FT; Oldenburg, AL; Marks, DL; Boppart, SA (2007). "Optical coherence tomography: a review of clinical development from bench to bedside.". Journal of biomedical optics 12 (5): 051403. Bibcode:2007JBO....12e1403Z.doi:10.1117/1.2793736. PMID 17994864.
- ^ A. F. Fercher, C. K. Hitzenberger, W. Drexler, G. Kamp, and H. Sattmann, " In Vivo Optical Coherence Tomography," Am. J. Ophthalmol., vol. 116, no. 1, pp. 113-114. 1993.
- ^ Swanson, E. A.; Izatt, J. A.; Hee, M. R.; Huang, D.; Lin, C. P.; Schuman, J. S.; Puliafito, C. A.; Fujimoto, J. G. (1993). "In vivo retinal imaging by optical coherence tomography". Optics Letters 18 (21): 1864–6. Bibcode:1993OptL...18.1864S.doi:10.1364/OL.18.001864. PMID 19829430.
- ^ Drexler, Wolfgang; Morgner, Uwe; Ghanta, Ravi K.; Kärtner, Franz X.; Schuman, Joel S.; Fujimoto, James G. (2001)."Ultrahigh-resolution ophthalmic optical coherence tomography". Nature Medicine 7 (4): 502–7.doi:10.1038/86589. PMC 1950821. PMID 11283681.
- ^ Kaufman, S; Musch, DC; Belin, MW; Cohen, EJ; Meisler, DM; Reinhart, WJ; Udell, IJ; Van Meter, WS (2004). "Confocal microscopy*1A report by the American Academy of Ophthalmology". Ophthalmology 111 (2): 396–406.doi:10.1016/j.ophtha.2003.12.002. PMID 15019397.
- ^ Riederer, S.J. (2000). "Current technical development of magnetic resonance imaging". IEEE Engineering in Medicine and Biology Magazine 19 (5): 34–41. doi:10.1109/51.870229.PMID 11016028.
- ^ M. Born and E. Wolf (2000). Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light. Cambridge University Press. ISBN 0-521-78449-2.
- ^ a b Fercher, A. F.; Mengedoht, K.; Werner, W. (1988). "Eye-length measurement by interferometry with partially coherent light". Optics Letters 13 (3): 186–8.Bibcode:1988OptL...13..186F. doi:10.1364/OL.13.000186.PMID 19742022.
- ^ Schmitt, J.M. (1999). "Optical coherence tomography (OCT): a review". IEEE Journal of Selected Topics in Quantum Electronics 5 (4): 1205. doi:10.1109/2944.796348.
- ^ a b Fercher, A (1995). "Measurement of intraocular distances by backscattering spectral interferometry". Optics Communications 117: 43. Bibcode:1995OptCo.117...43F.doi:10.1016/0030-4018(95)00119-S.
- ^ "Micromachined 2-D scanner for 3-D optical coherence tomography". Sensors and Actuators A: Physical 117 (2): 331. 2005. doi:10.1016/j.sna.2004.06.021.
- ^ Dunsby, C; Gu, Y; French, P (2003). "Single-shot phase-stepped wide-field coherencegated imaging". Optics express 11(2): 105–15. Bibcode:2003OExpr..11..105D.doi:10.1364/OE.11.000105. PMID 19461712.
- ^ Roy, M; Svahn, P; Cherel, L; Sheppard, CJR (2002). "Geometric phase-shifting for low-coherence interference microscopy". Optics and Lasers in Engineering 37 (6): 631.Bibcode:2002OptLE..37..631R. doi:10.1016/S0143-8166(01)00146-4.
- ^ Akiba, M.; Chan, K. P.; Tanno, N. (2003). "Full-field optical coherence tomography by two-dimensional heterodyne detection with a pair of CCD cameras". Optics Letters 28 (10): 816–8. Bibcode:2003OptL...28..816A.doi:10.1364/OL.28.000816. PMID 12779156.
- ^ Dubois, A; Vabre, L; Boccara, AC; Beaurepaire, E (2002). "High-resolution full-field optical coherence tomography with a Linnik microscope". Applied optics 41 (4): 805–12.Bibcode:2002ApOpt..41..805D. doi:10.1364/AO.41.000805.PMID 11993929.
- ^ Bourquin, S.; Seitz, P.; Salathé, R. P. (2001). "Optical coherence topography based on a two-dimensional smart detector array". Optics Letters 26 (8): 512–4.Bibcode:2001OptL...26..512B. doi:10.1364/OL.26.000512.PMID 18040369.
- ^ Dörr, Jan; Wernecke, KD; Bock, M; Gaede, G; Wuerfel, JT; Pfueller, CF; Bellmann-Strobl, J; Freing, A; Brandt, AU; Friedemann, P (2011 Apr 8). "Association of retinal and macular damage with brain atrophy in multiple sclerosis.". PloS one 6(4): e18132. doi:10.1371/journal.pone.0018132.PMID 21494659. Retrieved 21 November 2012.
- ^ Keane, PA; Patel, PJ; Liakopoulos, S; Heussen, FM; Sadda, SR; Tufail, A (2012 Sep). "Evaluation of age-related macular degeneration with optical coherence tomography.". Survey of ophthalmology 57 (5): 389–414. PMID 22898648.
- ^ WJ Walecki et al., Determining thickness of slabs of materials, US Patent 7,116,429, 2006
- ^ Wojtek J. Walecki and Fanny Szondy,"Integrated quantum efficiency, reflectance, topography and stress metrology for solar cell manufacturing", , Sunrise Optical LLC, Proc. SPIE 7064, 70640A (2008); doi:10.1117/12.797541
- ^ Wojciech J. Walecki, Kevin Lai, Alexander Pravdivtsev, Vitali Souchkov, Phuc Van, Talal Azfar, Tim Wong, S. H. Lau and Ann Koo, "Low-coherence interferometric absolute distance gauge for study of MEMS structures", Proc. SPIE 5716, 182 (2005);doi:10.1117/12.590013
- ^ Walecki, W. J., Lai, K., Souchkov, V., Van, P., Lau, S. and Koo, A. (2005), Novel noncontact thickness metrology for backend manufacturing of wide bandgap light emitting devices.physica status solidi (c), 2: 984–989.doi:10.1002/pssc.200460606
- ^ Wojciech Walecki, Frank Wei, Phuc Van, Kevin Lai, Tim Lee, S. H. Lau and Ann Koo, "Novel low coherence metrology for nondestructive characterization of high-aspect-ratio microfabricated and micromachined structures", Proc. SPIE 5343, 55 (2004); doi:10.1117/12.530749
- ^ Guss, G.; Bass, I.; Hackel, R.; Demos, S.G. (November 6, 2007). "High-resolution 3-D imaging of surface damage sites in fused silica with Optical Coherence Tomography". Lawrence Livermore National Laboratory UCRL-PROC-236270. Retrieved December 14, 2010.
- ^ W Walecki, F Wei, P Van, K Lai, T Lee, Interferometric Metrology for Thin and Ultra-Thin Compound Semiconductor Structures Mounted on Insulating Carriers, CS Mantech Conference, 2004
- ^ Wojciech J. Walecki, Alexander Pravdivtsev, Manuel Santos II and Ann Koo, "High-speed high-accuracy fiber optic low-coherence interferometry for in situ grinding and etching process monitoring", Proc. SPIE 6293, 62930D (2006);doi:10.1117/12.675592
- ^ see for example ZebraOptical Optoprofiler Probe
- ^ Wojtek J. Walecki and Fanny Szondy, "Fiber optics low-coherence IR interferometry for defense sensors manufacturing", SOLLC, Proc. SPIE 7322, 73220K (2009);doi:10.1117/12.818381
- ^ Dufour, Marc; Lamouche, G.; Gauthier, B.; Padioleau, C.; Monchalin, J.P. (2006). "Inspection of hard-to-reach industrial parts using small diameter probes". SPIE - The International Society for Optical Engineering. doi:10.1117/2.1200610.0467. Retrieved December 15, 2010.
- ^ Dufour, M. L.; Lamouche, G.; Detalle, V.; Gauthier, B.; Sammut, P. (April 2005). "Low-Coherence Interferometry, an Advanced Technique for Optical Metrology in Industry". Insight - Non-Destructive Testing and Condition Monitoring 47 (4): 216–219.doi:10.1784/insi.47.4.216.63149. ISSN 1354-2575. edi
Subscribe to:
Comments (Atom)









.JPG)









![I = k_1 I_S + k_2 I_S + 2 \sqrt { \left ( k_1 I_S \right ) \cdot \left ( k_2 I_S \right )} \cdot Re \left [\gamma \left ( \tau \right ) \right] \qquad (1)](http://upload.wikimedia.org/math/e/b/b/ebb2e079243f1472874e8233fc48ae22.png)
![\gamma \left ( \tau \right ) = \exp \left [- \left ( \frac{\pi\Delta\nu\tau}{2 \sqrt{\ln 2} } \right )^2 \right] \cdot \exp \left ( -j2\pi\nu_0\tau \right ) \qquad \quad (2)](http://upload.wikimedia.org/math/4/f/3/4f3aa72fd00ba105b77ef126d3e430aa.png)





